When a patient’s own respiratory drive falters, or when the lungs are simply too overwhelmed by disease to adequately exchange life-sustaining gases, the machine takes over. This is the essence of mechanical ventilation, an intervention that is neither a cure nor a simple pump, but rather a complex, life-support system that aims to bridge a period of acute respiratory failure while protecting the very delicate lung tissue. Understanding the fundamentals of this technology moves far beyond simply setting a rate and a volume; it requires a deep appreciation for the subtle, and often adverse, interaction between positive pressure and the body’s entire physiological landscape, particularly the cardiovascular system. The complexities involved in titration—the precise, moment-to-moment adjustment of settings—are less about following a rigid protocol and more about interpreting the nuanced mechanical signals emanating from the patient’s lungs and integrating those signals with the clinical picture of the entire individual.
The Complexities Involved in Titration are Less About Following a Rigid Protocol
The complexities involved in titration—the precise, moment-to-moment adjustment of settings—are less about following a rigid protocol and more about interpreting the nuanced mechanical signals emanating from the patient’s lungs.
The core mechanics of a mechanical ventilator stand in stark opposition to spontaneous, negative pressure breathing. In a healthy person, the diaphragm descends, the chest cavity expands, and the resultant negative pressure inside the chest draws air into the lungs. A ventilator, however, forces air into the lungs by applying positive pressure at the airway opening, essentially reversing the natural physiological gradient. This reversal is the source of many of the intervention’s potential complications. The positive pressure does not stop at the alveoli; it is transmitted through the thin lung tissue to the intrathoracic space, where it compresses the great veins and the heart itself. This compression directly impedes venous return—the flow of blood back to the right atrium—leading to a reduction in preload and, consequently, a fall in cardiac output. For a patient who is already hemodynamically unstable, even small increases in positive pressure can tip the balance toward catastrophic hypotension, a critical consideration that must always override the goal of perfect gas exchange.
This Compression Directly Impedes Venous Return
Changes in intrathoracic pressure (ITP) and lung volumes during positive pressure ventilation (PPV) impact right ventricular (RV) filling and emptying with the potential to induce hemodynamic compromise.
The precise control of the inspiratory cycle is governed by the selected mode of ventilation, which dictates the control variable—either Volume or Pressure—and the targeting scheme. In Volume Control (VC), the clinician sets a fixed tidal volume (VT), and the ventilator pushes air until that volume is achieved, allowing the resulting airway pressure to vary based on the patient’s lung and chest wall characteristics, specifically their compliance and resistance. Conversely, in Pressure Control (PC), the clinician sets a fixed inspiratory pressure (Pinsp), and the volume delivered is the variable outcome, changing as the patient’s lung mechanics change. This critical decision between volume and pressure control is a primary element of respiratory management, representing a trade-off between guaranteeing a minimum minute ventilation (VT) and ensuring that peak pressures do not exceed a level that risks inducing barotrauma or volutrauma. Changes in intrathoracic pressure (ITP) and lung volumes during positive pressure ventilation (PPV) impact right ventricular (RV) filling and emptying with the potential to induce hemodynamic compromise.
A Single-Flow Conducting Tube Representing the Airways
Pressure, volume, and flow as functions of time are related by a mathematical model called the Equation of Motion for the respiratory system.
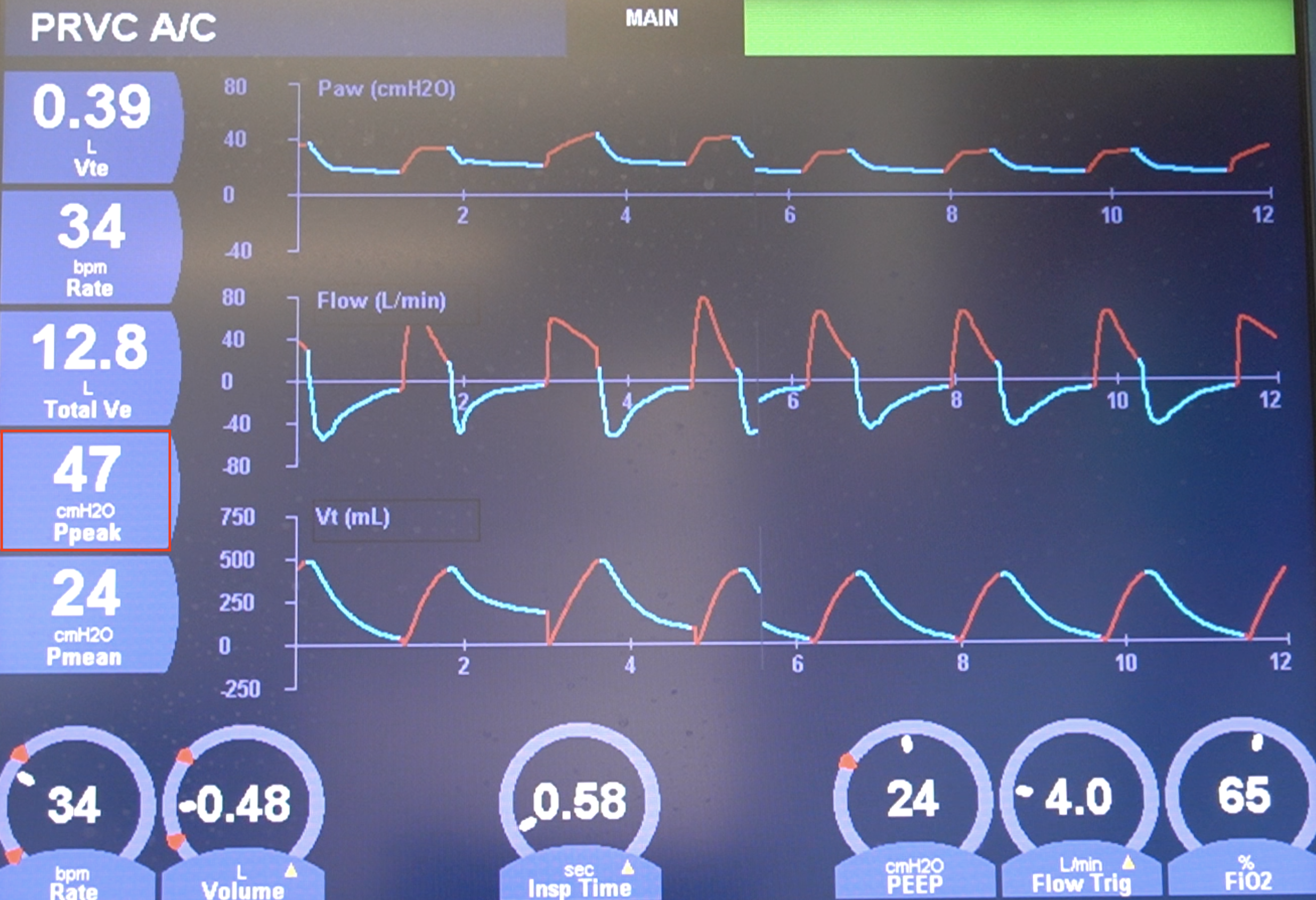
To accurately manage a patient on the ventilator, one must understand the Equation of Motion for the respiratory system. This mathematical model relates the pressure applied at the airway opening to the flow, resistance, volume, and compliance of the patient’s respiratory system. It conceptualizes the lung as a simple system composed of a single-flow conducting tube representing the airways and a single elastic compartment representing the alveoli and chest wall. Analyzing the components of this equation—specifically the resistive load (flow multiplied by resistance) and the elastic load (volume divided by compliance)—allows the clinician to interpret the subtle patterns displayed on the ventilator’s pressure-time and flow-time waveforms. For example, a sudden spike in Peak Inspiratory Pressure (Ppeak) without a corresponding change in Plateau Pressure (Pplat) points definitively toward an increase in airway resistance (perhaps due to bronchospasm or mucus plugging), not a decrease in lung compliance, thereby directing the immediate therapeutic intervention.
Plateau Pressure: The Estimation of Static Alveolar Pressure
In ventilated patients, Pplat is measured during an end-inspiratory pause (zero flow) and can be used to estimate total compliance.
The accurate measurement of specific pressures is non-negotiable for lung-protective ventilation. The Plateau Pressure (Pplat) is measured during an end-inspiratory pause—a brief moment when gas flow is zero, and all pressure is thus purely elastic pressure. This value is critically important as it provides the best clinical estimation of static alveolar pressure, the primary driving force for volutrauma (lung injury caused by excessive volume). The current standard of care for most forms of acute respiratory failure, particularly Acute Respiratory Distress Syndrome (ARDS), insists that Pplat be kept below 30 cmH2O to mitigate the risk of ventilator-associated lung injury (VALI). Furthermore, the Driving Pressure (ΔP or Pplat−PEEP), which is the pressure required to distend the lung from its end-expiratory to its end-inspiratory state, has emerged as an even stronger predictor of mortality than the plateau pressure alone, cementing the understanding that mechanical stress on the lung is a dynamic phenomenon. In ventilated patients, Pplat is measured during an end-inspiratory pause (zero flow) and can be used to estimate total compliance.
PEEP: Positive End-Expiratory Pressure
PEEP is the alveolar pressure above the atmospheric pressure at end-expiration.
The concept of Positive End-Expiratory Pressure (PEEP) is fundamental to contemporary mechanical ventilation. PEEP is the alveolar pressure above the atmospheric pressure at end-expiration. Its primary physiological function is to prevent the tiny, unstable air sacs (alveoli) from collapsing completely at the end of the breath. By keeping these units open—a process termed recruitment—PEEP facilitates better oxygenation by maintaining a higher Functional Residual Capacity (FRC) and preventing the detrimental process of atelectasis and the high shear stresses that occur when collapsed alveoli are repeatedly opened and closed. While low levels of PEEP (3 to 5 cmH2O) are routinely used to counterbalance the pressure loss from the endotracheal tube, higher levels are often required in severe lung disease to maximize oxygenation. However, high PEEP has a non-linear relationship with cardiac output; too much PEEP can significantly raise the Mean Airway Pressure (Pmean), further compressing the heart and potentially reducing oxygen delivery to the tissues despite an improved arterial oxygen saturation, a paradox that demands careful bedside assessment.
Synchronize the Patient’s Own Breathing Mechanism
There are mechanisms that can help synchronize the patient’s own breathing mechanism with the ventilator’s rhythm.
Ventilators have evolved sophisticated triggering mechanisms to prevent the machine from operating independently of the patient. The goal is to synchronize the patient’s own breathing mechanism with the ventilator’s rhythm, thereby promoting comfort and reducing the work of breathing. The two main triggers are flow trigger and pressure trigger. A flow trigger senses a small, rapid drop in the flow of gas within the circuit as the patient attempts to inhale, signaling the ventilator to immediately deliver a breath. A pressure trigger senses a slight, patient-initiated drop in the pressure within the circuit. The sensitivity of this trigger is a crucial setting; if it is too high, the patient has to exert excessive effort to start the breath, leading to fatigue. If it is too low, the ventilator may auto-trigger, delivering breaths unnecessarily and contributing to air trapping or dynamic hyperinflation, another major complication that elevates intrathoracic pressure and compromises hemodynamics.
Prolonging the Dying Process
If someone is unlikely to recover from their condition, putting them on mechanical ventilation may draw out the dying process.
Mechanical ventilation, despite its technological complexity, is ultimately a life-sustaining treatment that is temporary in nature. The goal is always to eventually wean the patient off the machine, a process that must be approached with both scientific rigor and clinical judgment. Weaning protocols typically involve assessing the patient’s readiness using key physiological metrics, such as the Rapid Shallow Breathing Index (RSBI)—the ratio of respiratory frequency to tidal volume—which indicates the efficiency of their spontaneous breathing. However, the decision to ventilate or to continue ventilation carries profound ethical weight, particularly in cases of irreversible disease. If someone is unlikely to recover from their condition, putting them on mechanical ventilation may draw out the dying process, causing unnecessary suffering for both the patient and their family. This requires clear, compassionate communication and the integration of medical capability with the patient’s established wishes and overall prognosis, highlighting the human element at the heart of critical care.
Ventilator-Associated Lung Injury and Barotrauma
Barotrauma occurs when there is alveolar damage due to high pressures entering the lungs.
The physical dangers of positive pressure ventilation cannot be overstated. The two primary mechanisms of ventilator-induced lung injury (VILI) are volutrauma and barotrauma. Barotrauma occurs when there is alveolar damage due to high pressures entering the lungs. This can lead to macroscopic complications like pneumothorax (collapsed lung), pneumomediastinum, or subcutaneous emphysema, all of which are associated with high mortality rates. Volutrauma, however, is often more subtle, resulting from the excessive stretch caused by overly large tidal volumes, even if the absolute pressure remains acceptable. This mechanical stretch triggers an inflammatory cascade within the lung tissue, releasing cytokines that can spill into the systemic circulation, causing multi-organ dysfunction—a phenomenon known as biotrauma. Modern ventilation strategies are thus defined by lung-protective principles, primarily employing lower tidal volumes (typically 4 to 8 mL/kg of predicted body weight) to minimize both stretch and resulting inflammation.
The Impact of Mechanical Ventilation on Hemodynamics
Mechanical ventilators use positive pressure, unlike normal breathing, which uses negative pressure; as such, ventilators will always have some impact on hemodynamics.
A common pitfall is to manage the ventilator purely based on lung parameters without factoring in its systemic consequences. Mechanical ventilators use positive pressure, unlike normal breathing, which uses negative pressure; as such, ventilators will always have some impact on hemodynamics. The inverse relationship between intrathoracic pressure and blood return means that every change in PEEP or Pmean must be immediately followed by an assessment of the patient’s blood pressure, heart rate, and peripheral perfusion. For a patient with severe sepsis or cardiogenic shock, the ventilator settings might need to be compromised—accepting a slightly higher PaCO2 or lower PaO2 (permissive hypercapnia and hypoxemia)—to prioritize maintaining adequate cardiac output and systemic perfusion. Ventilator management is therefore a delicate, high-stakes negotiation between optimal lung mechanics and cardiovascular stability.
A Single Elastic Compartment Representing the Chest Wall and Lungs
The advantage of this model is that it allows for simple representation of the respiratory system in terms of the mechanical properties of resistance and compliance.
In the critical care environment, the ventilator is not simply a machine; it is an extension of the patient’s physiology, and its settings must be tailored to the specific pathology. For a patient with Asthma or COPD, the primary problem is high airway resistance, requiring longer expiratory times to prevent air trapping. For a patient with ARDS, the problem is low compliance (stiffness) and alveolar collapse, requiring higher PEEP and careful monitoring of driving pressure. The single compartment model of the lungs allows for simple representation of the respiratory system in terms of the mechanical properties of resistance and compliance, which ultimately guides the choice of ventilator mode and the critical settings that determine whether the intervention facilitates recovery or contributes to further injury. This is a practice that is art as much as it is science, relying on the clinician’s capacity to integrate raw data with their physical examination and clinical intuition.
